Any doctor who treats patients with ocular inflammation knows the usefulness of corticosteroids to calm an inflamed eye. Should inflammation extend to the macula, vision loss follows and if not promptly treated can lead to permanent visual disability. While mild, uncomplicated macular edema can be managed with topical steroids, macular edema caused by posterior disease processes such as vascular occlusion, diabetic retinopathy or non-infectious posterior uveitis is best treated with intravitreal steroid. Intravitreal triamcinolone 40mg/mL (Triesence) has a long-demonstrated ability to treat posterior inflammation although its therapeutic effect may only last 2-3 months.
Increasingly more common are sustained-release steroid intravitreal implants. The following article reviews current implants being used in the USA, their indications and important considerations in patients being treated with these novel therapies.
Ozurdex (dexamethasone intravitreal implant) 0.7ug is approved for diabetic macular edema, macular edema from vascular occlusion as well as non-infectious posterior uveitis. The implant is a cylindrical rod that slowly exudes medication treating macular edema over 4-6 months. Iluvien (fluocinolone acetonide intravitreal implant) 0.19mg has a similar shape and is also inserted in office with an inserting device. It is FDA approved to treat diabetic macular edema up to 3 years with 1 treatment. Yutiq (fluocinolone acetonide intravitreal implant) 0.18mg is very similar in appearance and design however its approval is only for chronic, non-infectious posterior uveitis.
The last implant, Retisert (fluocinolone aceonide intravitreal implant) 0.59mg is also approved to provide treatment of chronic, non-infectious posterior uveitis over 30 months. Unlike the other aforementioned implants that are inserted using an injecting device and float freely in the vitreous body, Retisert is surgically implanted through an incision in the eyewall and is sutured behind the lens.
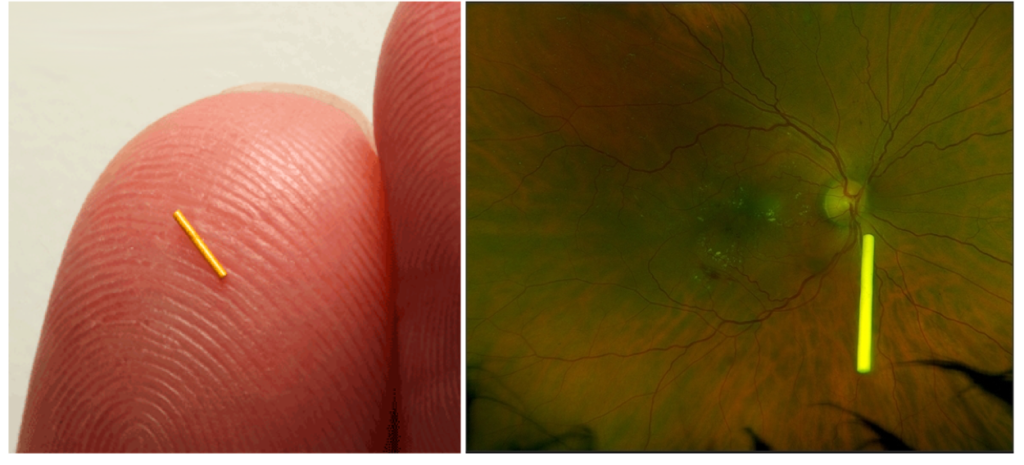
Figure 1: An Iluvien implant measuring 3.5mm x 0.37mm on the surface of the fingertip (left) and suspended in the posterior vitreous body (right).
In the case of chronic macular edema from diabetes or vein occlusion, our clinic has found use of these implants after the clinical failure of an anti-VEGF drug like Eyelea. Patients are generally enthused about the idea of fewer injections using longer acting implants like Iluvien, however, the potential side effects must be conveyed to patients beforehand.
Risks for endophthalmitis, migration of the implant into the anterior chamber and possible reactivation of herpetic disease must be discussed. All of the implants are expected to cause cataract formation in phakic patients. Increases in intraocular pressure are very common. The MEAD study that assessed the risk of IOP increases from dexamethasone implants found that around 25% of patients had a rise in ocular pressure <10mmHg, most of which were treatable with a single topical hypotensive1. Comparisons to large trials with fluocinolone delivery systems showed even greater increases in IOP; 34% of Iluvien patients saw an increase in IOP <10mmHg over the treatment period and 77% of Retisert patients were treated with IOP lowering medications2,3.
These trials revealed that most ocular hypertension was treated medically, however, 0.6% of Iluvien patients required incisional surgery to correct pressure, 1.2% of cases with repeated intravitreal triamcinolone (4mg), 4.8% of patients treated with Iluvien and 34-37% of patients treated with Retisert4.
Table 1: Comparison of Intravitreal Steroid Treatments
| Drug | Duration of Action (months) | Risk of IOP Increase 10mmg† or Need for IOP Lowering Medication* | Risk of Incisional Glaucoma Surgery |
| Triesence (triamcinolone 4mg)† | 2-3 | 20-60% | 0.6% |
| Ozurdex (dexamethasone 0.7ug)† | 4-6 | 25% | 1.2% |
| Iluvien (fluocinolone 0.19mg)† | Up to 36 | 34% | 4.8% |
| Retisert (fluocinoline 0.59mg)* | Over 30 | 77% | 34-37% |
In our clinic, we have found a use for steroid implants in a select group of chronically inflamed patients who have failed anti-VEGF drugs. We generally trial Triescene first to assess the patient’s intraocular pressure response before consideration of longer acting agents such as Iluvien. Many patients feel that if cataracts are inevitable and pressure can be controlled, they are accepting of an implant. It is always stressed that even after treatment, close monitoring is required.
References:
(1) Maturi, Raj K. MD; Pollack, Ayala MD; Uy, Harvey S. MD; Varano, Monica MD; Gomes, Andre M. V. MD; Li, Xiao-Yan MD; Cui, Harry MS; Lou, Jean MD; Hashad, Yehia MD; Whitcup, Scott M. MD for the Ozurdex MEAD Study Group INTRAOCULAR PRESSURE IN PATIENTS WITH DIABETIC MACULAR EDEMA TREATED WITH DEXAMETHASONE INTRAVITREAL IMPLANT IN THE 3-YEAR MEAD STUDY, Retina: June 2016 – Volume 36 – Issue 6 – p 1143-1152
(2) ILULIEN [Package Insert]. Alpharetta, GA: Alimera Sciences, Inc.; November 2016.
(3) RETISERT [Package Insert]. Rochester, NY: Bausch & Lomb Incorporated.; May 2019.
(4) Yonekawa, Y. & Wolfe, J; Dexamethasone Intravitreal Implant: Pharmacology and Clinical Update. Retina Today: September 2015

Author: Daniel T. Nolan, OD, FAAO
Specialty: Medical Eye Care
When treating patients suffering from chronic disease, chairside conversions generally involve new treatments available to help them. Working alongside Dr. Kristi Bailey in our medical retina service, I always look forward to educating patients and colleagues on new developments in retina. I am also grateful for the opportunity to help patients see better through co-managing with the greater optometric community.”









