Study Overview
Northwest Eye Surgeons is happy to announce that Drs. Cameron and Kuzin are Principal Investigators in an active FDA approved Phase III clinical study on iDose Travoprost Intraocular Implants. This is a prospective, randomized masked three year study comparing novel implantable glaucoma medication delivery devices to topical timolol. One of two biocompatible iDose device models are being inserted in ⅔ of the participants while ⅓ will remain the control arm and receive topical timolol. Completion of the precursory Phase II iDose trial was successfully and safely completed with all patients monitored for three years. Dr. Cameron along with Drs. Clermont, Bucher, and Jones are currently enrolling new patients in this nationwide study at the Northgate office who meet a specific list of required criteria. Dr. Kuzin along with Drs. Wright and Freeman are actively enrolling subjects in the Mount Vernon clinic. Participating patients receive a monetary stipend for their contribution to the study and complimentary glaucoma care for a three year period.
Who is Glaukos?
Glaukos Corporation is a pharmaceutical and ophthalmologic device manufacturer founded in 1998 dedicated to advancing the standard of care in treatment of glaucoma, corneal disorders and retinal disease. Glaukos is responsible for launching the first ever US-approved MIGS device, the iStent Trabecular Micro-Bypass stent in 2012 and the subsequent iStent inject in 2018.
What is the iDose Implant?
The iDose implant is a 1.8 x 0.5mm non-magnetic titanium intraocular device placed into the anterior chamber angle via a single 2.4mm corneal incision. When properly implanted the iDose is anchored into the sclera just behind the trabecular meshwork. The reservoir of the device containing travoprost extends into the anterior chamber. This allows for a slow therapeutic release of medication. Two different iDose implant models with varying elution rates are being utilized and compared in the study.
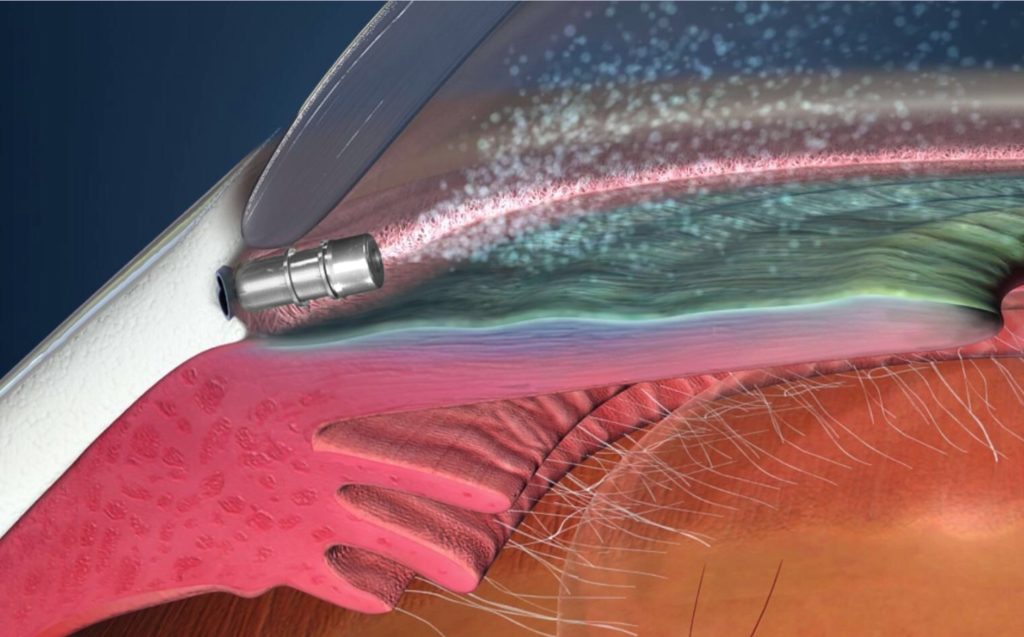
iDose implant anchored through the trabecular meshwork
Benefits of iDose implants include reducing ocular pressure by means other than eye drops. This permits therapeutic treatment without the adverse effects commonly accompanying topical medications such as allergic conjunctivitis and dry eye. Another benefit of this device includes fewer IOP fluctuations by eliminating eyedrop compliance hardships. If proper long term control is maintained, patients may require fewer incisional procedures requiring blebs which as we know carry their own set of risks.
Phase II Results
Preliminary results of the precursory Phase II trial as outlined by Jay Katz MD, Chief Medical Officer at Glaukos, showed good promise with iDose efficacy. Average ocular pressure reduction in the implanted cohort ranged from 7.9 to 8.5 mmHg over a twelve month period. This represented a 32-33% reduction in ocular pressure in the device study arm. The timolol-treated group required 31% more medications on average compared to the iDose cohort.
Study Criteria
In order to be considered for the Glaukos iDose GC-012 study, patients must meet the following inclusion requirements and be closely monitored during a ‘wash out period’ in which previously prescribed topical medications are discontinued:
- Age 18 or above with mild to moderate POAG or OHTN
- Phakic or Pseudophakic with PCIOL in the capsule
- Cup to Disc ratio of ≤0.8
- Prescribed less than 3 hypotensive topical medications
- BCVA 20/80 or better in each eye
- Visual field mean deviation no worse than -12dB
- No prior incisional glaucoma surgery and no prior SLT within 90 days
- No pending refractive or cataract surgery over a three year period

Magnified view of iDose device in nasal angle
Our Role As ODs
More reliable methods of glaucoma care are certainly under the research radar as evidenced by this Glaukos clinical study. With non-adherence to topical treatment often thwarting our best intentions, alternative dropless treatments such as laser trabeculoplasty and implantable drug delivery platforms warrant a future look as not only second line treatment but as initial treatment. Feel free to discuss this study further with any of your NWES glaucoma providers or to inquire if one of your patients may be an iDose implant candidate. Northwest Eye Surgeons remains committed to caring for our mutual patients at the highest level while supporting our profession’s push for improving the standard of eye care.

Author: Landon Jones, OD, FAAO
Specialties: Medical Eye Care
Seattle
As an optometric physician at NWES I pride myself on the opportunity to work side by side with our MD subspecialists. OD involvement in clinical research studies is another example of our collaborative approach to eye care and allows me to remain familiar with the most modern treatment modalities available to our patient population.









